For 12,000 years, there was a shortage of it. For 12,000 years, farmers spread all sorts of stuff on their fields in order to get more of this into their crops: from hay to oats, from blubber to blood, from seaweed to sea sponges, even human excrement. In some cities, farmers would even pay to be allowed to scoop out the contents of cesspits at night.
But in the last 100 years, the pendulum suddenly swung the other way. Instead of fighting a shortage, it’s now all hands on deck to deal with a surplus.
I’m talking about nitrogen, the crucial ingredient for agriculture.
How is it possible that we suddenly have a nitrogen problem, after thousands of years?
This has everything to do with the most important invention of the 20th century, which was made on 2 July 1909 by two Germans: Fritz Haber and Carl Bosch. They developed a process through which nitrogen from the air is combined with hydrogen in order to make synthetic ammonia: the raw material to make synthetic nitrogen fertiliser.
As a result, millions of people owe their lives to Haber and Bosch. The population explosion of the 20th century – from 1.6 billion people in 1900 to the 7.8 billion alive today – is in part thanks to them. At the same time, you can ask yourself whether the world is better off thanks to this invention. Because that enormous population growth – and the nitrogen released through the use of synthetic fertilisers – have been to the detriment of our natural environment.
It is, as founding editor Rob Wijnberg has previously written here, the great paradox of our time: everything is both better and worse than ever before.
And synthetic fertilisers play a crucial role in that story.
The oldest agricultural problem in the world
Just under 12,000 years ago, there was a fundamental change in humanity’s development. We slowly changed from itinerant hunter-gatherers to become sedentary farmers.
This was the first Agricultural Revolution: a great leap forward. For the first time, we were no longer dependent on what we found in the wild: we decided ourselves what should grow where.
But according to the world-famous historian Yuval Noah Harari, this isn’t progress at all. It is a luxury trap. “If the adoption of ploughing increased a village’s population from 100 to 110, which 10 people would have volunteered to starve so that the others could go back to the good old time? There was no going back. The trap snapped shut,” Harari writes.
That trap exists only because of nitrogen. From the moment that we started planting seeds in order to feed ourselves, our existence became inextricably tied up with the stuff. Anyone who did anything in agriculture in the thousands of years that followed had to ensure they had a steady supply of it.
The nitrogen problem is the oldest agricultural problem in the world.
Synthetic fertiliser, nitrogen, ammonia, what’s the story exactly?
Let’s start with the basics.
In agriculture, there are two simple rules. One: by harvesting, you remove nutrients from the soil, and they need to be replenished. Two: the yield of your land is determined by the nutrient that is most scarce. And that is almost always nitrogen – crucial for plant growth.
The reason the supply of nitrogen is complicated is that there are very few natural sources of nitrogen. At least, of usable nitrogen. Our atmosphere is full of nitrogen – in fact, it composes 80% of our air – but unfortunately we can’t use that. It’s dinitrogen (N2), an unreactive variant, which means it does not combine with other elements. Only a group of special bacteria, or lightning, can turn dinitrogen into the reactive variant that can be absorbed by plants through soil.
Of course we can find usable nitrogen in nature: it’s in manure, for instance. The sharp smell you get in areas with a lot of livestock is the natural variant of ammonia that comes from manure, which spreads nitrogen. The presence of nitrogen in plant remains is the reason why cereal stubble is burned on the field in traditional farming. The burned ashes replenish the nitrogen in the soil. Or farmers could opt for crop rotation: some crops, like certain legumes, can replenish the nitrogen content of the soil. But if you want large-scale harvests, all this does not yield enough nitrogen to replenish the loss after harvesting.
Guano mania
People have been searching for better forms of usable nitrogen for centuries. In 1804, the Prussian naturalist Alexander von Humboldt scraped some hardened bird poop from a rock off the coast of Peru and brought it back to Europe to study. It turned out that guano – as the bird poop is known – is a kind of super fertiliser. It contains up to 14% nitrogen.
And nitrogen is life.
This led to an age of guano mania – there was even a US law enacted in 1856 that allowed US Americans to annex uninhabited islands covered in bird poop: the Guano Islands Act. The original inhabitants of Peru, including the Inca, had been using guano for over 2,000 years by then. Killing or disturbing birds during breeding season was even punishable by death among the Inca.
With growing populations and disappointing agricultural yields, guano was of vital importance in many countries at the beginning of the 19th century. In the United States, the population doubled every 25 years, Benjamin Franklin observed in 1751. The British demographer Thomas Malthus used these developments to support his famous catastrophe theory: if the population grows faster than food production, that will irrevocably lead to famine and death, until the balance is restored.
Mining guano for nitrogen couldn’t avert such a catastrophe. By 1900, about 340,000 tonnes of nitrogen were extracted worldwide. That is only 2% of the amount of nitrogen that was lost through harvesting that year.
A solution had to be found, or famine would strike.
The most important invention of the 20th century
That solution was the Haber-Bosch process, a method that uses atmospheric nitrogen (N2) and hydrogen (H) to produce ammonia. The process breaks the triple bond between the two nitrogen atoms and binds each atom to three hydrogen atoms, which is ammonia.
I hear you thinking, “Yeah, so?”
Well, those bags of Miracle-Gro or lawn fertiliser you buy at the garden centre have nitrogen in them – not the natural, unreactive kind, but so-called ammonium nitrate: greyish-white pellets, largely made of synthetic ammonia.
The Haber-Bosch process takes the previously unusable nitrogen from the air and makes it possible to use it for all sorts of applications.
This sounds complicated. And it is. Yet, this is also the most important invention of the 20th century.
Haber and Bosch made their discovery at the Badische Anilin & Soda Fabrik in Germany, better known as BASF: the biggest chemical company in the world today. Chemist Fritz Haber invented the process. Carl Bosch – who was a metallurgist as well as a chemist – was responsible for upscaling. He is the one who managed to convince the BASF management to invest in the necessary equipment. “I believe it can go. I know exactly the capability of the steel industry. It should be risked,” he said at a meeting in March 1909.
Thanks to Haber, Bosch and Rossignol millions of people are having dinner tonight
A third name is rarely mentioned yet is just as important: Haber’s assistant Robert Le Rossignol. He was a British chemist who played a crucial role because he was also a skilled instrument maker. He designed the parts and built the prototype of the machine that produced the first drops of ammonia.
In scaling up, Haber, Bosch, Rossignol and their colleagues were forced to take a number of risks. What nature accomplishes with lightning – breaking the incredibly strong triple bond between two nitrogen atoms – can only be accomplished artificially at extremely high temperatures: about 1000 degrees celsius. The problem is that at that temperature, a large part of the formed ammonia is burned off. In order to achieve the same chemical reaction at lower temperatures an enormous increase in pressure is needed: a whopping 20 megapascal, about 100 times the amount of pressure in your bike tires.
Just to be sure, the three scientists poured their steel test tubes into reinforced concrete. And it’s a good thing they did, because they exploded the first time. Eventually, the chemists managed to combine various layers of softer and harder steel to make a tube strong and big enough to facilitate the process.
The result: usable nitrogen in fluid form. The key ingredient of synthetic fertilisers. The year was 1912.
The Haber-Bosch process spells an end to Malthus’s predictions. Thanks to Haber, Bosch and Rossignol millions of people are having dinner tonight. Haber received the Nobel Prize for this in 1918, because he turned air into bread.
Science saves the day.
Or, did it?
Synthetic ammonia isn’t a crucial ingredient of fertilisers only. The terrible explosion on 4 August in Beirut this year? That was 2,750 tonnes of ammonium nitrate: a crucial component of artificial fertilisers and explosives.
At the beginning of the first world war, Germany only had six months of nitrate stored and the British Marine was blocking the supply from Chile. But then along came Haber, Bosch and Rossignol. Without their ammonia synthesis, the first world war would not have lasted four years.
There won’t be many other people in history simultaneously responsible for both saving and ending so many lives.
Fertilisers are the anabolic steroids of agriculture
The production of ammonia skyrocketed, mainly in order to make synthetic fertiliser. Dutch farmers were first in line. In the interbellum between the two world wars, they were frontrunners in the innovative use of synthetic fertilisers, even more so than the Germans themselves.
Nowadays, the Haber-Bosch process supplies the entire world with all the nitrogen it needs, and because of this there is more than enough food. As a result, two things grew very quickly in the 20th century: the world’s population and the production of fertilisers. And those two are inextricably linked. Fertilisers are the anabolic steroids of agriculture.
In 1950, there were 2.5 billion people, and 2.6 million tonnes of ammonia were produced; by 2019 we had reached 7.8 billion people and 150 million tonnes of ammonia.
Progress is a one-way trip. And this is potentially disastrous
When you do the math, synthetic fertiliser production is highly efficient. Ammonia synthesis accounts for 1% of global energy consumption, 80% of that ammonia production is used for fertilisers, which in turn supplies 40% of the global food chain – and even 50% of the Chinese food chain.
Let that sink in for a minute. That means that of every five people on earth, at least two of them are alive thanks to fertilisers. In China that number is one in two.
There is a very simple conclusion we should draw from these numbers: we can’t do without synthetic fertilisers.
It’s the Agricultural Revolution of the 20th century, but just like the first one, 12,000 years ago, it’s a trap with no way out. Who’s going to volunteer to die of hunger so we can go back to a world without an excess of nitrogen?
Progress is a one-way trip. And this is potentially disastrous.

Why nitrogen is a ‘wicked problem’
The global population is predicted to reach 10 billion by 2050. This means we’re only going to need more synthetic fertilisers. And more synthetic fertiliser means more nitrogen.
That excess of nitrogen is disastrous for biodiversity. Plants need nitrogen to grow, but when there is too much of it certain plants that love nitrogen grow excessively. As a result, other more vulnerable plants are pushed out, and with them the birds, insects and other animals that are dependent on them also disappear. Thanks to Haber-Bosch, we have flooded our environment with a nutrient that is naturally scarce, thus throwing entire ecosystems out of balance.
But this excess of nitrogen is just the sour cherry on the cake. Nitrogen is not a normal problem; it’s a “wicked problem”.
Here’s why.
There are two cycles that are crucial to life on earth: the carbon cycle and the nitrogen cycle. The more these cycles are “managed” by humans, the more they seem to derail.
We have flooded our environment with a nutrient that is naturally scarce, thus throwing entire ecosystems out of balance
The carbon cycle has been completely disrupted by high levels of fossil fuel emissions. The same thing is happening to the nitrogen cycle. Unfortunately, these are not isolated problems. As is often the case, the world is much more complex than you want it to be.
The Haber-Bosch process has entangled the nitrogen cycle and the carbon cycle because the process relies on fossil fuels. In less technical terms: our potatoes grow on natural gas. And that means CO2 emissions.
That 1% of global energy consumption mentioned earlier may be efficient, but it still translates to an average of 2.8 tonnes of CO2 emissions per tonne of ammonia produced. For the 150 million tonnes of ammonia produced in 2019, that amounts to 420 million tonnes of CO2, more than 1% of global CO2 emissions.
The entanglement goes even further. Our means of transport, including those CO2-producing steel machines we call cars, emit nitrogen oxide. All that contributes to the nitrogen surplus. Since 1980, the world’s population, the amount of reactive nitrogen and carbon have been increasing one-to-one.
And yet, we are completely dependent on synthetic nitrogen fertilisers. After all, there are mouths to feed. How’s that for a Devil’s bargain: either contribute to ecological devastation or famine?
And we’re not there yet, unfortunately. As I said before, it’s a wicked problem. In addition to the nitrogen problem and the CO2 emissions from the production of ammonia, the Haber-Bosch process has led to a change in our diet. After all, we also need to look into what all that synthetic fertiliser is actually used for.
Who are we feeding with all those extra crops?
Luxury for everyone
Every year at Christmas time in the Netherlands, you see the same Lidl ad at bus shelters across the country: “Luxury for everyone” it reads, showing a plastic tray of fondue meat. You can read this in three different ways.
- As a call for a fair distribution of luxury. Which is great, of course.
- As a whopper of a contradiction in terms – luxury is exclusive by definition; that’s what makes it luxury.
- As an excellent summary of a huge problem captured in one simple poster: meat consumption as the norm.
In 2015, global meat consumption was 300bn kilos, a six-fold increase compared to 1950 (50bn kilos). Yet, the world’s population has “only” increased threefold. This means we’re eating much more meat than we used to, and that trend is not slowing down. Meat consumption is predicted to be at 450bn kilos by 2050.
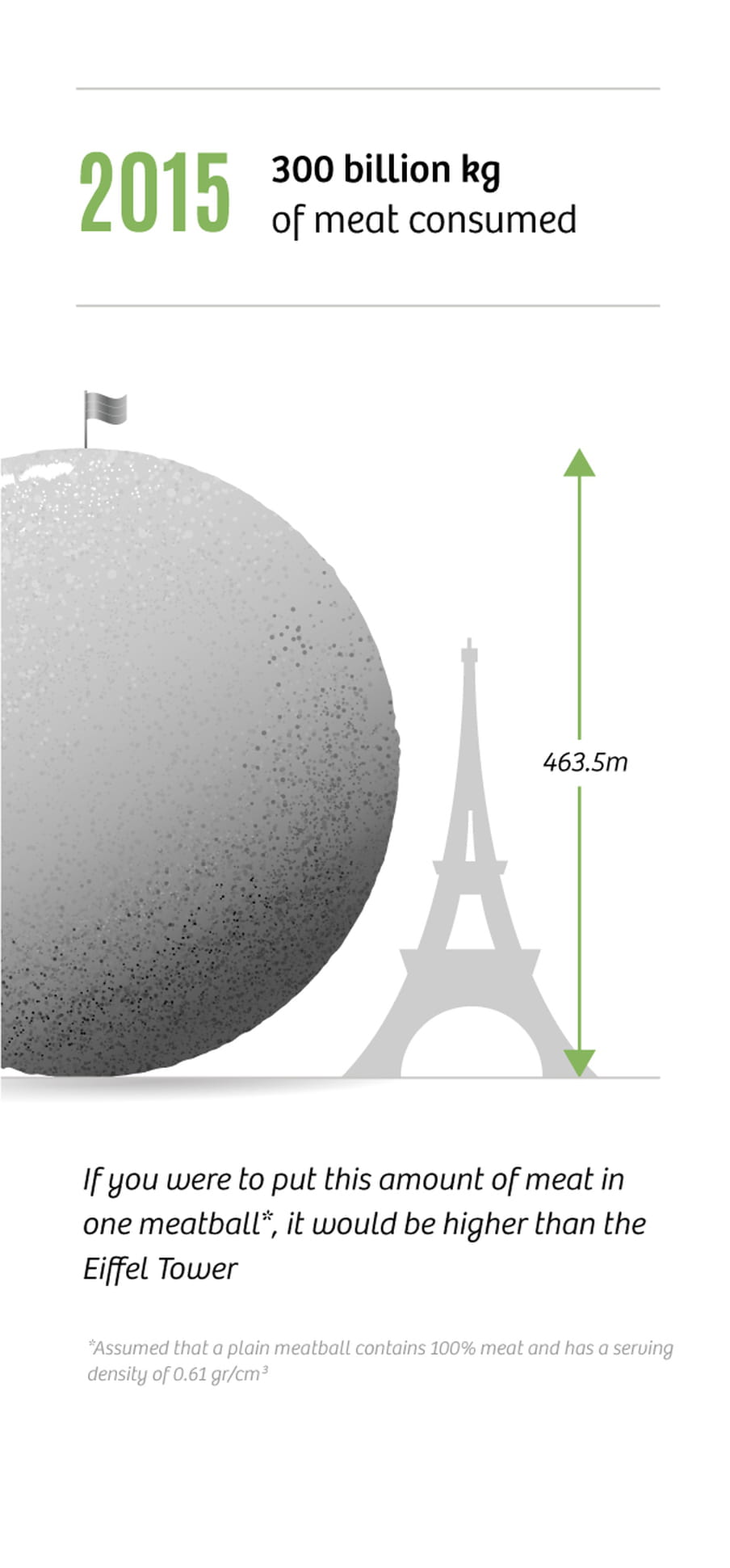
In countries like the US and Australia, the average person consumes more than 100kg of meat per year. The Netherlands is not far behind, with 75kg per capita. In general the rule is, the richer the more meat is consumed. By now, the combined weight of cows grazing our planet is up to 600m tonnes. By way of comparison, the combined weight of all the humans on earth is 370m tonnes. And all those animals need to be fed. This change in our diets is the reason we are so dependent on synthetic fertilisers.
We spread hundreds of thousands of kilos of synthetic fertilisers on our farmland to grow crops like maize, which are not for our own consumption, but that of our livestock. This accounts for about 50-60% of rich countries’ agricultural production. Worldwide, this number is 35%. Europe raises the average: at least 80% of European harvests are destined to become animal feed. The EU offers up a whopping 65% of its farmland for the production of animal feed.
Nearly 9% of the world’s population was undernourished in 2019. Because the rich want cheap meat
This huge number of livestock in turn produces its own CO2, methane and ammonia – and these figures are many times higher than the 1% of the Haber-Bosch process itself. That was only the first link in a chain of pollution. Breeding livestock is responsible for nearly 15% of worldwide greenhouse gas emissions.
And that’s without mentioning the gigantic water consumption that comes with breeding livestock, the deforestation required to make room for the farms, or the widespread suffering of animals.
These are times we live in. We’re producing more than enough food to feed 7.8 billion mouths. We could even feed 10 billion, but we’re not: nearly 9% of the world’s population was undernourished in 2019. Because the rich want cheap meat.
We don’t have a food supply problem; we have a food distribution problem.
Luxury for everyone means a liveable world for no one
The most obvious way to cut down on our use of synthetic fertilisers lies in what we eat.
Imagine the timespan of our existence – homo sapiens – on this planet as 24 hours. According to that analogy, we’ve only been practising agriculture for about an hour and a half – the last 12,000 years. All that time, the nitrogen problem was one of shortage. Then, in the very last minute of those 24 hours, we managed to make fertilisers using synthetic ammonia. In the last 30 seconds, production has increased by 800%. We use this to grow crops – not for ourselves, but to feed a livestock population that’s growing just as quickly, while also producing manure: ammonia, in other words. That is the nitrogen problem of 2020: overabundance in the extreme.
Should we be eating meat, then? No, not in these quantities.
Never before have we eaten meat in these absurd quantities, and certainly not beef. The simplest way to decrease the production of artificial fertilisers is, in fact, very simple: eat less meat. Even better would be to replace meat with beans, lentils and chickpeas. Legumes that can create their own nitrogen thanks to a special bacteria in their roots, which means they don’t need to be fertilised.

But how do you get people to eat less meat?
The easiest way is not by highlighting the devastating environmental impact, but by simply raising the price so that it is more in line with the actual costs: the demands it makes on energy consumption, the environment, water and the atmosphere.
But we know what we’d be letting ourselves in for. Instead of a few farmers blocking a motorway with their tractors, there would be a mass uprising. What has been made into a commodity cannot easily be brought back to being a luxury. That’s the luxury trap. And it’s not the first time we’ve fallen for it.
Luxury for everyone: that’s a liveable world for no one.
Translated from the Dutch by Hannah Kousbroek.
Dig deeper
 We can’t stop using steel. Here’s how we can make it the foundation of a new, sustainable iron age
We build everything from railroads to lunchboxes with steel, and for good reason: it’s strong, flexible and easily mass produced. But the iron production necessary to create steel is majorly polluting the planet. And it’s almost impossible to replace the material because we need it to keep building. So how do we move forward?
We can’t stop using steel. Here’s how we can make it the foundation of a new, sustainable iron age
We build everything from railroads to lunchboxes with steel, and for good reason: it’s strong, flexible and easily mass produced. But the iron production necessary to create steel is majorly polluting the planet. And it’s almost impossible to replace the material because we need it to keep building. So how do we move forward?